Research Highlights
Non-magnetic cations direct magnetism
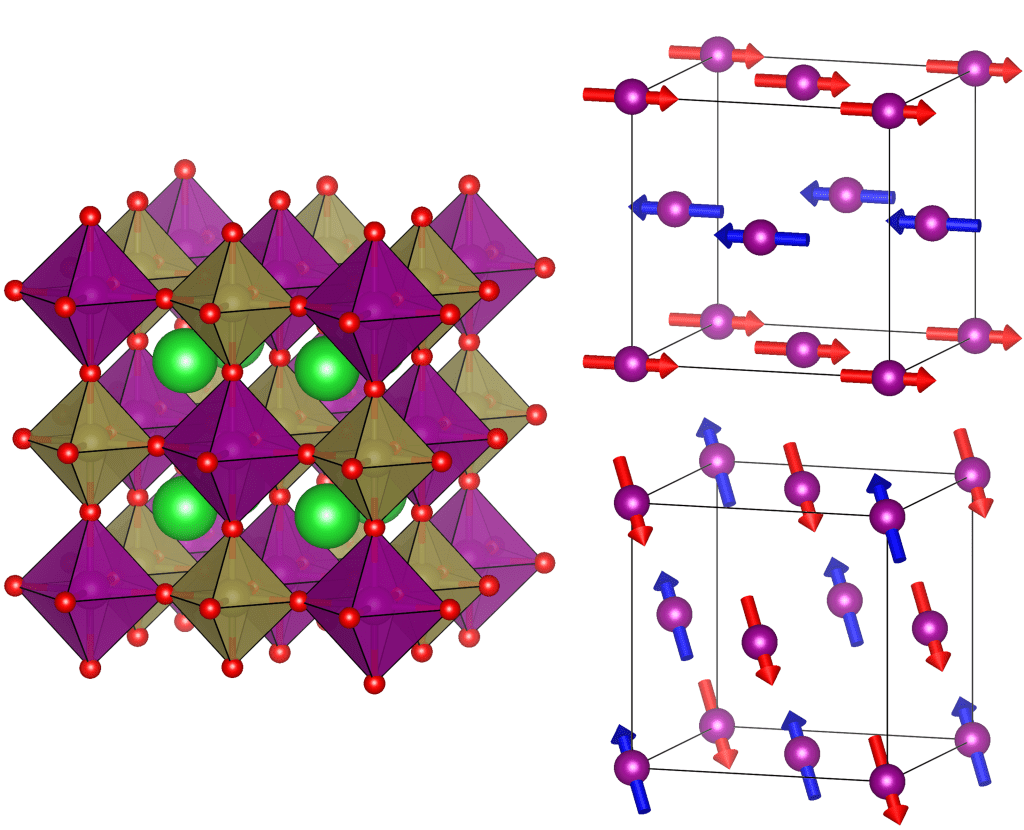
Magnetism in solids arises from tiny magnetic moments known as spin. These are typically located on magnetic cations with unpaired electrons such as Mn2+ (3d5, S = 5/2) or Cu2+ (3d9, S = 1/2). At low temperatures, the spins stop moving and form an ordered magnetic structure. The magnetic ordering is driven by the magnetic interactions between the spins.
It is generally thought that non-magnetic cations do not have an effect on the magnetic interactions. We investigated two manganese double perovskites Ba2MnTeO6 and Ba2MnWO6, where the magnetic Mn2+ cations are connected by non-magnetic Te6+ (d10) or W6+ (d0) cations. We were able to show that the non-magnetic connecting cation has a significant effect on the interactions: d10 cations favor nearest-neighbor interactions and d0 cations favor next-nearest-neighbor interactions. This d10/d0 effect is general to double perovskites, where the magnetic cation is a 3d transition metal.
Read more on the ISIS Science Hightlights.
Read the articles in Chemistry of Materials and Physical Review Materials.
Quantum spin liquid on the square lattice
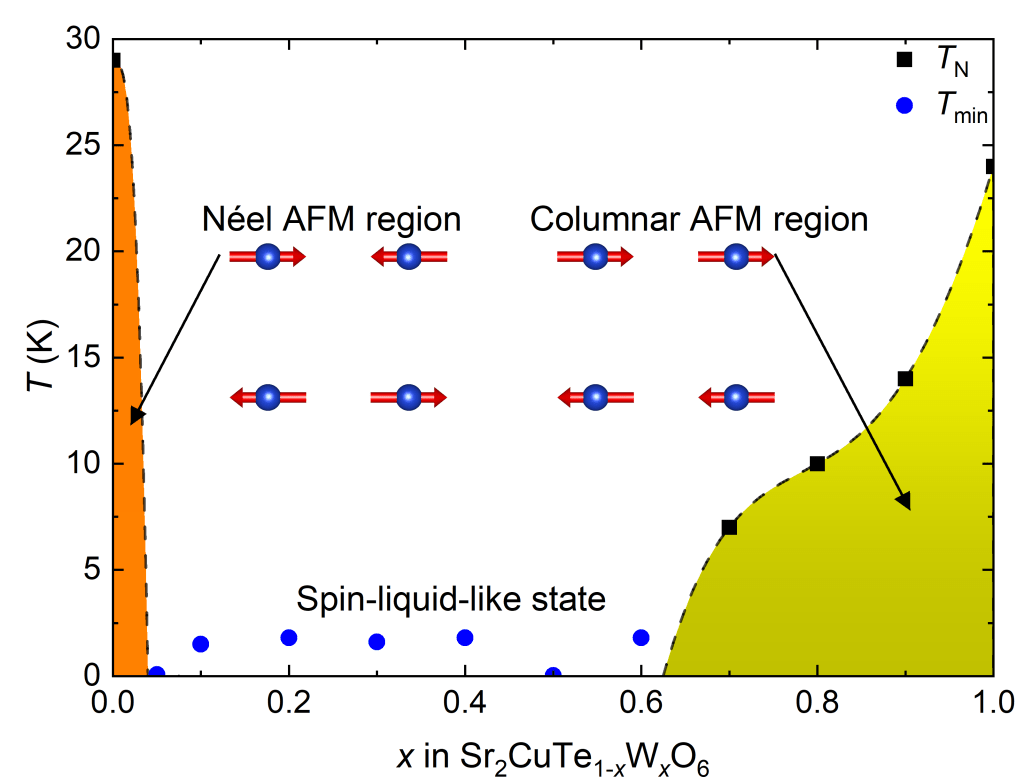
In conventional magnetic materials, the tiny magnetic moments – spins – order at low temperatures. Quantum spin liquids are exotic materials, where the spins do not order or freeze even at absolute zero. These materials could be used as the building blocks of next-negeration quantum computers. Nobel Laureate Philip W. Anderson proposed in 1987 that a quantum spin liquid could exist on a square lattice of S = 1/2 cations such as Cu2+. However, this type of quantum spin liquid has never been observed.
We investigated the properties of Cu2+ double perovskites Sr2CuTeO6, Sr2CuWO6, and their solid solution Sr2CuTe1−xWxO6. Curiously, the magnetically ordered parent phases exist on opposite sides of the of the predicted quantum spin liquid state. We were able to show that a spin-liquid-like state forms in the Sr2CuTe1−xWxO6 solid solution in a wide composition range of x = 0.05−0.6. This is the first observation of a spin-liquid-like state on the square lattice.
Read more on Phys.org.
Read the articles in Nature Communications, Chemistry of Materials, Physical Review Letters and Physical Review B.
Valence bond glass

Magnetism in 4d and 5d transition metal compounds can be complex, since the cations have an orbital moment in addition to the spin moment. In some materials these become entangled together to form pseudospins such as Jeff = 3/2 or Jeff = 1/2. The interactions between the pseudospins are complicated and can result in unusual quantum states.
One example is the valence bond glass state found in Ba2YMoO6, where the Mo5+ Jeff = 3/2 states pair up to form non-magnetic singlets (“valence bonds”) in a glassy manner. We showed that a valence bond glass state also forms in Ba2LuMoO6, but without a spin freezing transition as found in Ba2YMoO6. This makes Ba2LuMoO6 an ideal material for exploring valence bond glass physics.
Read more on the ISIS Science Highlights.
Read the article in npj Quantum Materials.